Fluzone is the commercial name of an influenza virus vaccine, distributed by Sanofi Pasteur. It is the most commonly used adult flu shot in the US.
February 2019 – Immunogenicity and reactogenicity of high- vs. standard-dose trivalent inactivated influenza vaccine in healthcare workers: a pilot randomized controlled trial “We did not have information on influenza infection history, which may also alter response to vaccination. Self-reported previous vaccination may have been subject to recall bias, although self-report has been found to be a sensitive and fairly specific indicator of true vaccine status, particularly in younger adults. We studied the impact of previous season vaccination relative to one season’s vaccination and three vaccine strains and our data may not be generalizable to other seasons or viral strains.”
May 01, 2018 – FLUZONE® Quadrivalent Influenza Virus Vaccine Quadrivalent Types A and B (Split Virion) 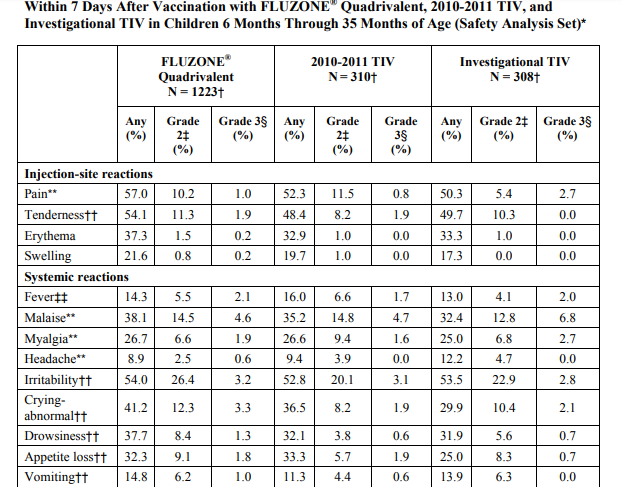
February 18, 2017 – Fluzone HD vs SD cluster randomized trial in US NHs 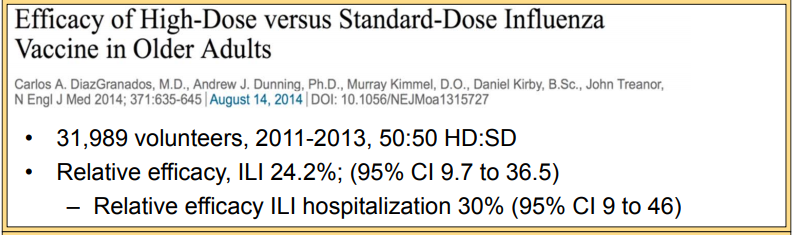
August 12, 2016 – Fluzone® High-Dose Influenza Vaccine “IIV3-HD is a trivalent, inactivated, split-virus influenza vaccine containing 60 µg HA of each influenza strain, which is four times the HA content of standard-dose influenza vaccines.
October 11, 2013 – Immunogenicity and safety of Fluzone® intradermal and high-dose influenza vaccines in older adults ≥65 years of age: A randomized, controlled, phase II trial (full text) “For all vaccines, most solicited reactions were generally mild or moderate and resolved within 3–7 days (data not shown). Injection-site reactions were reported by similar proportions of older adult subjects receiving the 15 μg (76.5%) or 21 μg (77.3%) ID vaccines, but they were reported more often by subjects immunized with the ID vaccines than by those receiving the HD (49.5%) or SD (34.5%) IM vaccines (Table 5). Among SD vaccine recipients, the proportion reporting injection-site reactions was higher for younger adults (64.3%) than for older adults (34.5%).”
March 2, 2012 – Febrile seizures after 2010–2011 influenza vaccine in young children, United States: A vaccine safety signal from the vaccine adverse event reporting system “On November 23, 2010, the combination of the coding term “febrile convulsion” and the Fluzone® TIV product exceeded a predetermined threshold in the VAERS database. By December 10, we confirmed 43 reports of febrile seizure following TIV in children aged 6–23 months. Clinical features of most reports were consistent with typical uncomplicated febrile seizures, and all children recovered. Further epidemiologic assessment of a possible association between TIV and febrile seizures was undertaken in a separate, population-based vaccine safety monitoring system.”
January 2012 – Fluzone(®) Intradermal vaccine: a promising new chance to increase the acceptability of influenza vaccination in adults. “Obtaining optimal acceptability of intradermal vaccines may represent an additional asset to help increase the coverage of influenza vaccination in young adults.”
May 19, 2011 – FDA Fluzone, Fluzone High-Dose and Fluzone Intradermal Supporting Documents
May 9, 2011 – FDA Approval Letter – Fluzone Intradermal FDA Licenses Fluzone Intradermal – “We are waiving the pediatric study requirement for this application because this product does not represent a meaningful therapeutic benefit over existing therapies for pediatric patients and is not likely to be used in a substantial number of pediatric patients. Available data indicate that the immune response to inactivated influenza vaccines in infants <6 months of age is not as robust as in older children due to immaturity of the immune response and interference from maternal antibody. For the age group 6 months through 17 years, Fluzone Intradermal provides no meaningful therapeutic benefit over licensed influenza vaccines and is not likely to be used in a substantial number of infants increased risk for local reactogenicity and children because of the .”
February 24, 2011 – Preliminary Data: Febrile Seizures Increased With Fluzone Plus Prevnar 13 “Concern about Fluzone – the only influenza vaccine recommended for use for the 2010-2011 flu season in infants and children 6-23 months of age – arose from a data mining signal from the passive Vaccine Adverse Events Reporting System. Prior to that, a report from Australia suggested a statistically increased risk for febrile seizures within a day of receipt of another brand of flu vaccine that has since been pulled from the market worldwide.”
January 20, 2011 – Fluzone Vaccine Safety FDA and CDC Update on Fluzone Influenza Vaccine and VAERS Reports of Febrile Seizures in Children
October 27, 2010 – A Synthetic Adjuvant to Enhance and Expand Immune Responses to Influenza Vaccines (full text) “These data suggest that the virus-specific recall responses can be modulated by formulating Fluzone; inclusion of an oil-in-water emulsion induces a TH2 response whereas the addition of a TLR4 agonist to the emulsion directs a TH1 response.”
July 14, 2010 – Seroprevalence Following the Second Wave of Pandemic 2009 H1N1 Influenza in Pittsburgh, PA, USA “Our finding of high anti-2009 H1N1 seroprevalences among school children and high anti-1918 H1N1 seroprevalences among the elderly suggest that further viral transmission is not likely.”
April 30, 2010 – Licensure of a High-Dose Inactivated Influenza Vaccine for Persons Aged ≥65 Years (Fluzone High-Dose) and Guidance for Use — United States, 2010 “Standard dose inactivated trivalent influenza vaccines contain a total of 45 µg (15 µg of each of the three recommended strains) of influenza virus hemagglutinin antigen per 0.5mL dose (5). In contrast, Fluzone High-Dose is formulated to contain a total of 180 µg (60 µg of each strain) of influenza virus hemagglutinin antigen in each 0.5mL dose.”
March 15, 2010 – Safety and Efficacy Study of Fluzone® Vaccine Combined With Different Doses of JVRS-100 Adjuvant (H-100-001) Purpose: This study is designed to assess safety, tolerability and immunogenicity of Fluzone® vaccine with four dose levels of JVRS-100 adjuvant compared to Fluzone® vaccine alone in healthy adults 18-49 years of age.
March 10, 2010 – Phase IV Trial to Collect Safety Data and Sera for Immunogenicity Testing in Healthy Children Given Fluzone® Vaccine
January 27, 2009 – Safety and Immunogenicity of Inactivated Influenza Virus Vaccine Among Healthy Children 6-12 Weeks of Age (GRC28)
April 2008 – Anaphylaxis from the Influenza Virus Vaccine “The patient’s IgE immunoblot showed a protein band at 100 kDa which is similar to the molecular weight of gelatin protein, a 68-kDa protein which is similar to the molecular weight of hemagglutinin protein from the influenza vaccine, and a 45-kDa protein band that is similar to the molecular weight of ovalbumin protein from chicken embryo/egg. Conclusion: Based on clinical symptoms, skin testing, Immunocap testing and immunoblot evaluation, we feel that our patient is allergic to the infectious agent in the influenza vaccine as well as gelatin and ovalbumin in egg.”
March 2007 – Higher antibody, but not cell-mediated, responses to vaccination in high physically fit elderly (full text)
September 12, 2002 – FDA approves preservative-free influenza vaccine for pediatric use


