Flucelvax is a flu vaccine made from dog kidney cells. Flucelvax is now approved for people aged 18 and older.
Flucelvax Vaccine Package Insert
“FLUCELVAX (Influenza Virus Vaccine), a vaccine for intramuscular injection, is a “subunit” influenza virus vaccine prepared from virus propagated in Madin Darby Canine Kidney (MDCK) cells, a continuous cell line. These cells were adapted to grow freely in suspension in culture medium. The virus is inactivated with ß-propiolactone, disrupted by the detergent cetyltrimethylammonium bromide and purified through several process steps. Each of the 3 virus strains is produced and purified separately then pooled to formulate the trivalent vaccine.”
October 11, 2019 – Cell-Based Flu Vaccines “Flucelvax Quadrivalent is the only cell-based inactivated influenza vaccine that has been licensed by the FDA for use during the 2019-2020 flu season. … With BARDA support, two vaccines licensed in the US are produced in cell culture: recombinant influenza vaccine (RIV, Flublok™) manufactured in insect cells and inactivated mammalian cell-grown vaccine (ccIIV, Flucelvax™).
October 9, 2018 – Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: a step towards improved influenza vaccine effectiveness “Although circulating A(H3N2) viruses did not differ antigenically from that recommended by the WHO for vaccine production, overall interim vaccine effectiveness estimates were below historic averages (33%) for A(H3N2) viruses.”
November 27, 2015 – Surveillance of adverse events after the first trivalent inactivated influenza vaccine produced in mammalian cell culture (Flucelvax®) reported to the Vaccine Adverse Event Reporting System (VAERS), United States, 2013–2015 Note: This table of adverse events is from the full text. 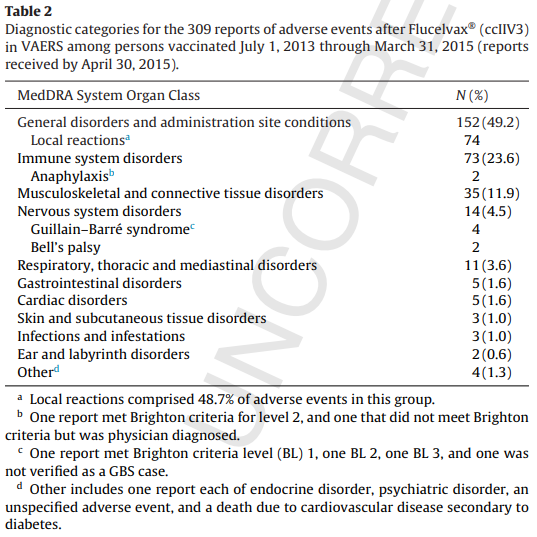
April 2013 – Egg-independent influenza vaccine: making great strides forward (pdf) “On November 20, 2012, the US FDA approved its first cell derived seasonal influenza vaccine made by Novartis for adults 18 years old and older. The vaccine, Flucelvax, is a trivalent, subunit, inactivated influenza vaccine for seasonal use and was approved by the European Union in 2007 under the trade name of Optaflu. Unlike conventional egg-based manufacturing, the seed viruses of Flucelvax were cultivated in a Madin Darby Canine Kidney (MDCK 33016-PF) cell line that has been adapted for growth in suspension in a closed fermenter system under serum-free, protein-free conditions, which is associated with a reduced risk of contamination and avoiding generation of egg-adaptive mutations in hemagglutinin (HA).”
January 7, 2013 – Cell-based vaccines yield only modest advances for seasonal flu “In late November, the US Food and Drug Administration approved the first influenza vaccine grown in cell culture in the country: Novartis’s Flucelvax, a seasonal flu vaccine that is produced in dog kidney cells. Another company, Protein Sciences of Meriden, Connecticut, expects to receive US approval for its recombinant flu vaccine—Flublok, which uses an engineered virus that expresses flu proteins when it replicates within insect cells—by mid-January. (In Europe, a version of Flucelvax and a vaccine made in monkey kidney cells from Illinois-based Baxter are already available.)”
January 1, 2013 – Flucelvax: First seasonal vaccine using cell-culture technology “Flucelvax’s approval was based on data from a clinical trial conducted in the United States and Europe involving 11,404 people 18 years to 49 years old (mean age 33 y). Patients were randomized in a 1:1:1 ratio to receive either Flucelvax (n = 3,828); Agriflu, an egg-based seasonal influenza vaccine also produced by Novartis (n = 3,676); or a placebo (n = 3,900). Results from the trial showed that Flucelvax was 83.8% effective in preventing influenza compared with placebo.”


